Furoscix is a unique, subcutaneous furosemide injection developed by scPharmaceuticals Incorporated, a company dedicated to the advancement of cardiorenal healthcare. This injectable diuretic received standard FDA approval on October 10, 2022 for the at-home treatment of congestion in patients with chronic heart failure. Following its supplemental New Drug Application (sNDA) approval on March 6, 2025, Furoscix can now be further used to treat edema in patients with chronic kidney disease, including nephrotic syndrome.
Empty space, drag to resize
Chronic kidney disease is a common but taxing condition, and the CDC states that more than
1 in 7 U.S. adults (~ 35.5 million people) are affected by it. Further, as many as 9 out of 10 adults are unaware that they even have chronic kidney disease. With these overwhelming statistics, it is important for both patients and healthcare providers to be well-versed and proactive in managing this condition.
In short, chronic kidney disease involves progressive kidney damage and is primarily characterized by the inability to properly or completely filter blood in the body. As a result, many complications can arise if the disease progresses or is left untreated. Common
complications of chronic kidney disease include the following:
Empty space, drag to resize
Nephrotic syndrome is unique in its relation to chronic kidney disease because it can either cause the disease or manifest as a result of it. On its own, nephrotic syndrome is a kidney disorder caused by damage to the glomeruli, or the filtering units of the kidneys. This condition is characterized by excessive protein loss in the urine (proteinuria), low blood protein levels (hypoalbuminemia), edema, and high cholesterol levels (hyperlipidemia). Albumin helps anchor fluid in the bloodstream, so with low albumin levels many of these patients experience severe edema.
The major mechanisms of edema include sodium retention, increased capillary pressure, and hypoalbuminemia, which lead to fluid overload and leakage into interstitial spaces. Edema can often be seen in patients with chronic kidney disease, as the kidneys are unable to effectively eliminate sodium and water from the body. This accumulation of excess body fluid is commonly seen in the lower extremities of patients with chronic kidney disease, but it can also occur in the hands, face, and in extreme cases, the lungs.
The management of edema in chronic kidney disease is one of the best ways to prevent serious complications, improve patient wellbeing, and slow disease progression. The next few sections will discuss some of the different complications of unmanaged edema and why it is important to treat as soon as possible.
Excess body fluid is linked to increases in blood volume and pressure, which places additional strain on the heart. Due to this, edema can contribute to hypertension, left ventricular hypertrophy, and congestive heart failure (CHF) if left untreated. By controlling fluid retention, patients can reduce their cardiovascular burden and lower their risk of heart-related complications.
When edema grows severe or is left untreated, excess fluid can accumulate in the lungs through alveoli leakage. This overload can impair natural gas exchanges in the lungs, causing difficulty breathing, low oxygen levels, and life-threatening respiratory distress. Proper edema management with diuretics and fluid restriction can help prevent this dangerous condition and ensures better respiratory function.
Accelerated Kidney Damage
While it might go without saying, it is important to note that poorly managed edema can accelerate kidney damage. As mentioned above, excess fluid retention leads to increased sodium and water retention, elevating pressure within the kidneys and contributing to irreversible damage. By maintaining proper fluid balance, patients can reduce stress on their kidneys and potentially slow the progression of CKD.
Diuretics play a crucial role in the management of edema by helping the body to eliminate excess fluid and sodium, which reduces swelling and prevents complications. In using diuretics to promote fluid control, healthcare providers can help patients maintain better cardiovascular health, kidney function, and overall well-being. Taking proactive measures to manage fluid overload can significantly improve health outcomes and reduce the burden of CKD-related complications.
Furoscix is a subcutaneous injection formulation of the loop diuretic furosemide. This unique formulation comes as a pre-filled, on-body drug delivery system that allows patients to receive continuous furosemide without hospital admission. Furosemide is a commonly used diuretic for treating edema and hypertension, with other dosage forms including tablets, oral liquid, and intravenous solutions.
Furosemide works by inhibiting the Na+/K+/2Cl- co-transporter in the thick ascending limb of the loop of Henle in the kidneys.
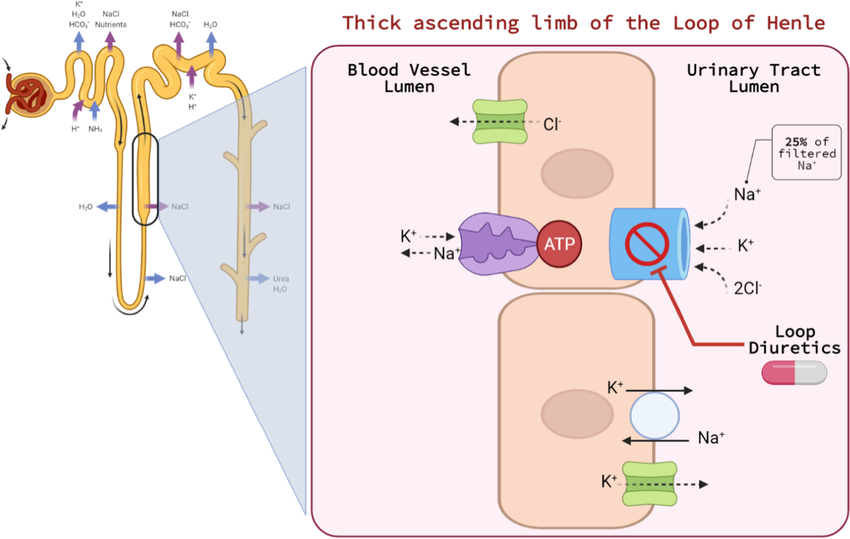
This ion transporter plays a crucial role in the reabsorption of sodium, potassium, and chloride ions from the urine back into the bloodstream. By blocking this transporter, furosemide prevents the reabsorption of these ions, leading to an increase in the excretion of sodium, chloride, water, and other electrolytes like potassium and calcium. Increased urine production reduces fluid buildup and mitigates the risks associated with unmanaged edema.
Furoscix is available as a single-dose continuous injection device. Furoscix contains 80mg/10mL of furosemide, dispensing 30mg in the first hour followed by 12.5mg/hour for a total of up to 5 hours. While furoscix can work to quickly restore fluid balance in patients with edema, scPharmaceuticals is sure to emphasize that Furoscix should not be used daily and should be replaced by oral diuretics as soon as possible to avoid excessive fluid loss. It can also be noted that if the full 80mg of Furoscix is not used in one dose, per provider orders, the device can be stored at room temperature for up to 2 years for further use.
Furoscix received its
initial FDA approval in October of 2022, with the support of an open-label, crossover study of
subcutaneous versus intravenous furosemide. In this clinical study, Furoscix showed equivalence in diuresis and bioavailability compared to IV furosemide, supporting Furoscix as an alternative treatment option for patients that require diuresis but cannot easily access inpatient IV treatment.
This first wave of approval, however, was only for the indication of chronic heart failure congestion. After submitting a supplemental new drug application (sNDA), the FDA has now
further approved Furoscix for treating edema in chronic kidney disease and nephrotic syndrome.
“Expanding the [furosemide injection] indication to include patients with chronic kidney disease will provide a much-needed additional tool for clinicians to utilize in our management of fluid overload,” said Suneel Udani, consulting physician at Nephrology Associates of Northern Illinois and Indiana (NANI). “Utilizing [furosemide injection] can potentially help us keep our patients with heart failure and/or CKD at home while we restore and maintain euvolemia.”
John Tucker, Chief Executive Officer of scPharmaceuticals, also stated that "In anticipation of this approval, [scPharmaceuticals has] taken strategic steps to ensure a successful launch, including key opinion leader engagement, comprehensive market research, and commercial readiness initiatives. [scPharmaceuticals is] excited to introduce FUROSCIX to nephrologists and [is] focused on providing treatment options to both heart failure and CKD patients experiencing acute fluid overload."
This new indication approval underscores the pharmaceutical industry’s dedication to filling in the gaps of patient care. Ceuticon champions innovation and is devoted to providing actionable insights that can help professionals lead change in the healthcare industry. By staying up to date on current events like Furoscix’ label expansion, we empower healthcare professionals to utilize new developments for improved patient outcomes.