Initially approved by the FDA in October 2023, Omvoh was the first drug of its class to be approved for the treatment of moderately to severely active ulcerative colitis (UC) in adults. Omvoh was first approved via the standard approval pathway by way of a Biologics License Application (BLA).
As of January 15, 2025, Omvoh is now further approved for the treatment of moderate to severe Crohn’s disease (CD) in adults.
Empty space, drag to resize
Crohn’s disease is a chronic inflammatory bowel disease that affects the gastrointestinal (GI) tract. While it mainly affects the end of the small intestine and the beginning of the colon, Crohn’s disease can ultimately attack any part of the GI tract.
The inflammation associated with Crohn’s disease can be increasingly painful and even debilitating to patients if left untreated. Symptoms like abdominal pain, fatigue, diarrhea, and weight loss may be commonly seen in patients with Crohn’s disease, and flare-ups may also occur if the condition is not well managed.
While Crohn’s disease is not curable, remission is possible and remains the main goal of Crohn’s disease treatment. Other therapeutic goals include inflammation reduction, symptom relief, and flare-up prevention.
"The burden of Crohn's disease on patients' daily lives is substantial," said Michael Osso, president and CEO, Crohn's & Colitis Foundation, about Omvoh’s new indication. "This approval is meaningful for adult patients with Crohn's disease, who now have more treatment options available."
Empty space, drag to resize
Eli Lilly's Omvoh is a biologic drug that works directly in the GI tract to combat inflammation caused by inflammatory bowel disease in adults. It is not yet known if Omvoh is safe and effective in children less than 18 years of age.
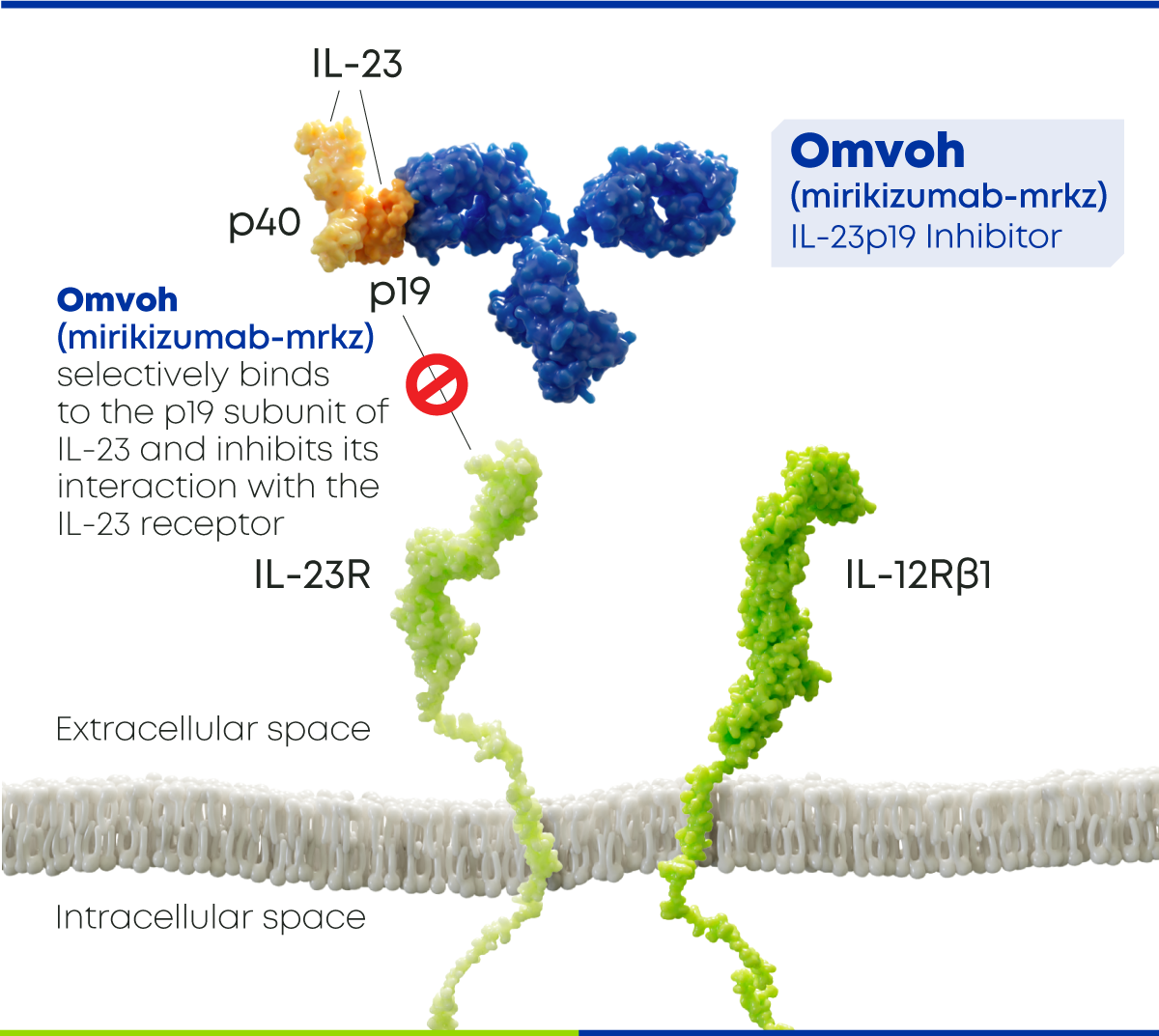
Interleukin-23 (IL-23) is a pro-inflammatory cytokine that contributes to intestinal inflammation. Due to this, overactivity of the IL-23 pathway plays a critical role in the development of inflammatory bowel diseases like ulcerative colitis and Crohn's disease. Omvoh (mirikizumab-mrkz) is an interleukin-23p19 antagonist that specifically targets the p19 subunit of IL-23, thereby inhibiting the IL-23 pathway.
Omvoh is utilized to induce and maintain remission in patients with Crohn’s disease. General information regarding the
dosing and administration of this medication can be found below:
-
Induction Therapy: Omvoh is available as a 300mg/15mL single-dose vial for intravenous infusion; patients must receive this medication at a healthcare facility every 4 weeks for their first 3 doses (infusion lasts about 90 minutes)
-
Maintenance Therapy: Available as a 100mg/mL or 200mg/2mL formulation in a prefilled pen or prefilled syringe; patients should self-administer the medication as a subcutaneous injection once every 4 weeks
The current landscape for treating Crohn’s disease consists of a variety of medications that aim to reduce inflammation, mitigate symptoms or flares, and/or induce then maintain remission.
The
Crohn’s & Colitis Foundation is a widely recognized non-profit organization that provides research, advocacy, and education to both patients and health professionals about the two major types of inflammatory bowel disease (Crohn’s disease and ulcerative colitis). The foundation provides a detailed list of medications for treating Crohn’s disease on its website, which can be viewed
here. Below is a brief overview of the primary drug classes used in Crohn’s disease treatment, including biologics such as Omvoh.
Hence the name, this class of drugs contains 5-aminosalicyclic acid as the major active ingredient. While this class of drugs is not yet approved by the FDA for treatment in Crohn’s disease, their off-label use may still be seen often in its treatment. These medications decrease inflammation in the lining of the GI tract, working best in the colon but not much in the small intestine.
Off-label aminosalicylates are generally used for mild to moderate episodes of Crohn’s disease and can be used as a maintenance therapy to prevent flare-ups or relapses in the disease. Common drugs in this class include the following:
Corticosteroids are non-selective immunosuppressive agents that are often used in the treatment of moderate to severe Crohn’s disease. As a non-selective agent, these medications suppress the entire immune response rather than specific pathways that cause GI inflammation. This widespread immune suppression can significantly increase the risk of infections in patients. As a result, the American Gastroenterological Association (AGA) guidelines recommend limiting corticosteroid use to short-term treatment for specific signs and symptoms in moderate to severe Crohn’s disease.
Corticosteroids often used in patients with Crohn’s disease include:
-
Prednisone
-
Methylprednisolone
While corticosteroids suppress the immune system, immunomodulators for IBD decrease immune responses to prevent ongoing inflammation in the gastrointestinal tract. These medications are not indicated for the induction of remission but may be used as monotherapy or as part of a combination for the maintenance of remission in patients with moderate to severe Crohn’s disease. Immunomodulators can also reduce or eliminate the need for corticosteroid use in these patients.
Immunomodulators mentioned in the
AGA guidelines for Crohn’s disease treatment include:
-
Azathioprine
-
6-mercaptopurine
Biologic medications are often reserved for patients that have not responded to conventional therapies for Crohn’s disease. Eli Lilly’s Omvoh (mirikizumab) falls into this category of medications that work by preventing specific proteins from contributing to inflammation in the GI tract. While Omvoh is an IL-23 inhibiting biologic, other biologics, like
anti-TNF (anti-tumor necrosis factor) and
integrin receptor antagonist medications are also common choices. Some common biologics used to treat Crohn’s disease include:
-
-
Certolizumab pegol
-
Adalimumab
-
Infliximab
-
Natalizumab
-
Vedolizumab
-
Ustekinumab
To support the new indication approval for Omvoh, the
Phase 3 VIVID-1 study was conducted as a randomized, placebo-controlled trial in adults with moderate to severe Crohn’s disease. Participants included those who experienced a loss of response, inadequate response, or intolerance to corticosteroids, immunomodulators, and/or other biologic therapies.
The primary endpoints of this study with their respective statistics are as follows:
-
Clinical remission: defined as a Crohn’s Disease Activity Index (CDAI) score of less than 150 at one year of treatment
- 53% of patients on Omvoh versus 36% of patients on placebo achieved these results at one year
-
Endoscopic response: defined as visible healing of the intestinal lining via endoscopy and > 50% reduction from baseline in Simple Endoscopic Score for Crohn’s Disease (SES-CD) score
- 46% of patients on Omvoh versus 23% on placebo met this endpoint after one year
- 32% of Omvoh patients achieved early endoscopic response at just three months of treatment, compared to 11% of patients on placebo
*Note that the placebo group included patients that were switched to Omvoh after 12 weeks if they had no self-reported clinical response; 40% of the initial placebo group was switched to Omvoh after the first 12 weeks.
The most common adverse reactions associated with Omvoh throughout this trial were upper respiratory tract infections (28%), injection site reactions (10%), and headaches (6%).
Omvoh is currently being evaluated in the ongoing, open-label extension (OLE) VIVID-2 study, which aims to analyze its efficacy and safety in adults with moderate to severe CD for up to three years of treatment. Participants in the VIVID-2 study completed 52 weeks of treatment in the VIVID-1 study, and the primary objective is to evaluate the long-term effects of mirikizumab in these patients. So far, over 80% of patients have maintained endoscopic responses and nearly 90% of patients have maintained clinical remission with two years of continuous Omvoh treatment.
Omvoh has already been FDA-approved for Ulcerative Colitis. With this, Omvoh already has utilization management requirements for Ulcerative Colitis, including one or more steps through other biologic or biosimilar products and/or a minimum 3-month trial of conventional therapy (e.g. aminosalicylates, corticosteroids or immunomodulators). Additionally, some health plans require a gastroenterology specialist to prescribe the medication.
While utilization management practices are yet to be discovered for Omvoh in patients with Crohn’s disease, Eli Lilly announced that Omvoh had gained first-line biologic coverage from two major pharmacy benefit managers (PBM) as of January 1, 2025. This means that under those PBMs, Omvoh is available on formulary in the preferred specialty tier and does not require failure of other biologics prior to use. Lilly also offers patient support programs for Omvoh that offers co-pay cards and other assistance to eligible patients.
Eli Lilly shares that they have also submitted marketing applications for Omvoh in Crohn’s disease in the European Union and Japan, as they aim to achieve global access. For ulcerative colitis, Omvoh is currently approved in 44 countries worldwide.
The FDA’s approval of Omvoh for Crohn’s disease marks another step forward in the management of inflammatory bowel diseases for both patients and providers.
"Many patients with Crohn's disease have tried available therapies and are still seeking a treatment option that can work well for them to help control their disease," explained Marla Dubinsky, M.D., chief, division of pediatric gastroenterology and nutrition, co-director, Susan and Leonard Feinstein IBD Clinical Center, Mount Sinai Kravis Children's Hospital, Icahn School of Medicine, Mount Sinai New York. "The FDA approval of Omvoh may help adults with Crohn's disease achieve long-term remission and visible healing of the intestinal lining, even if they have tried other medications that did not work or stopped working."
"People living with Crohn's disease have shared with us how truly disruptive symptoms such as abdominal pain, frequent bowel movements and bowel urgency can be," said Daniel M. Skovronsky, M.D., Ph.D., chief scientific officer, and president of Lilly Research Laboratories and Lilly Immunology. "With Omvoh approved in both Crohn's disease and ulcerative colitis, more patients now have a treatment option that may provide long-term disease control and address key symptoms that matter most to them, reflecting Lilly's ongoing commitment to elevate care and improve outcomes for patients."
The approval of Omvoh for Crohn’s disease highlights the ever-evolving nature of the pharmaceutical landscape and the vital role healthcare professionals play in translating these advancements into better patient outcomes. At Ceuticon, we strive to bridge the gap between innovation and implementation by providing pharmaceutical professionals and pharmacists with the education and resources they need to stay ahead. By fostering a deeper understanding of therapies like Omvoh, we empower professionals to make a meaningful impact on patient care.